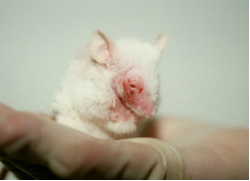
It took 20 years of campaigning and several delays, but on March 11, 2013, the European Union law went in to effect to ban cosmetic products and their ingredients that use research on animals. The ban stipulates that no new products tested on animals will be imported in the EU. This ban will include products from skin cream and toothpaste to bleaches and air fresheners. The EU ban follows a similar import ban in Israel that went into effect in early January for toiletries, detergents, and cosmetics. These import bans mean that any company that uses animal testing for new cosmetics will no longer be able to sell those products in the EU or Israeli markets.
The EU cosmetic industry is estimated to be worth $91 billion per year. The import ban opens the question as to whether or not other countries will end up adopting the EU standard in order to compete in the market. India is one country that recently made some movement in response to the ban. In late February, Drug Controller General of India, Dr. GN Singh ordered animal testing for cosmetics to be indefinitely suspended. In addition, an elected representative, Baijayant Panda, is also urging India to follow the EU and Israel to set an example for cruelty-free products.
On the other hand, in China, the law mandates that manufacturers test products on animals before selling to consumers. This will put China at an economic disadvantage since they will not be able to sell those cosmetic items in Europe. However, the Chinese regulation also puts outside brands in a bind. If they wish to sell within China, it will require the companies to make exceptions to comply with the required animal testing. A company claiming to be cruelty-free but allowing Chinese regulators to test certain products on animals could tarnish a company’s image in the eyes of its consumers. For example, the Japanese cosmetic company Shiseido, responded to the EU ban by explaining that in April, it would only use animal testing in rare instances of safety concerns or for import to China.
The Humane Society describes some of the types of animal tests used for researching cosmetic chemicals include skin and eye irritation tests used on rabbits without pain relief, force-feeding tests to look for cancer or birth defects, and lethal dose tests that feed chemicals to animals to discover the dosage level that causes death. However, the Physicians Committee for Responsible Medicine consider animal testing alternatives to be necessary because animal research is not effective and does not accurately predict the way the human body will react to chemicals it is given.
The European Commission has been researching alternatives since 1991 but formally established the European Union Reference Laboratory with the launch of the European Centre for the Validation of Alternative Methods in 2011 to work towards reducing and replacing animals for chemical, biological, and vaccination safety testing. Animal alternatives include human volunteers for clinical studies, synthetic human tissue, computer simulation programs, or in vitro cell cultures. From 2007 to 2011, the European Commission made 238 million euros available to fund the research of alternative methods.
Despite the progress made, some people disagree with this view that alternatives are the better choice. Scientist Jennifer Sass of the Natural Resources Defense Council believes the alternatives cannot address all of the safety issues present. For example, synthetic skin tests will not indicate detrimental effects to the immune system. Furthermore, some scientists still firmly believe animal testing can be useful for humans in areas such as genetics, stem cell research, and the development of antibiotics and other pharmaceuticals. One German lawmaker, Ms. Roth-Behrendt, also believes the EU ban for cosmetics still provides companies with a loophole. She theorizes that if the ingredients tested on animals for non-cosmetic purposes, such as pharmaceuticals, the cosmetics companies could potentially use them. In addition, ingredients tested on animals prior to the ban will remain on the shelves. The EU remains firm on the ban however in hopes that scientists and regulators will collaborate and create innovative alternative methods.
It will be interesting to see whether other countries such as the U.S., Canada, Russia, or Brazil will adopt the EU standard to ban animal testing with cosmetics or if more corporations will voluntarily react to the changed law like Shiseido. Groups like PETA will likely continue to work closely on advocating for change in China; especially as science and technology progresses, animal advocates will hope that in time the laws will reflect a more humane method of research and development. It is possible that this EU ban will push more movement toward banning animal testing, not just for cosmetics, but also in other areas of safety testing such as medicine and pharmaceuticals.
Kristen Pariser is a 2L and Staff Editor for the Denver Journal of International Law and Policy.

